Integrity of the human genome is protected by surveillance mechanisms that coordinate the cell cycle progression and DNA repair. In the presence of DNA damage, cells temporarily arrest in the cell cycle checkpoint to prevent transmission of mutations to progeny and continue proliferation after completing DNA repair. The checkpoint and DNA repair are tightly interconnected by signalling cascades that involve protein phosphorylation (ATM, ATR, CHK1/2, CDK1, PLK1 kinases), ubiquitination (BRCA1, RNF168, 53BP1) and gene expression (tumour suppressor protein p53). Deficient cell cycle checkpoints or impaired DNA repair allow proliferation in the presence of damaged DNA, promoting genome instability and eventually malignant transformation. In our laboratory, we employ cell and molecular biology approaches, CRISPR-mediated gene editing and transgenic mouse models to investigate how cells respond to DNA damage. We also seek for genetic defects in cancer cells that could be exploited for personalized cancer treatment.
Topic 1. Role of PPM1D/WIP1 in DNA damage response and oncogenesis
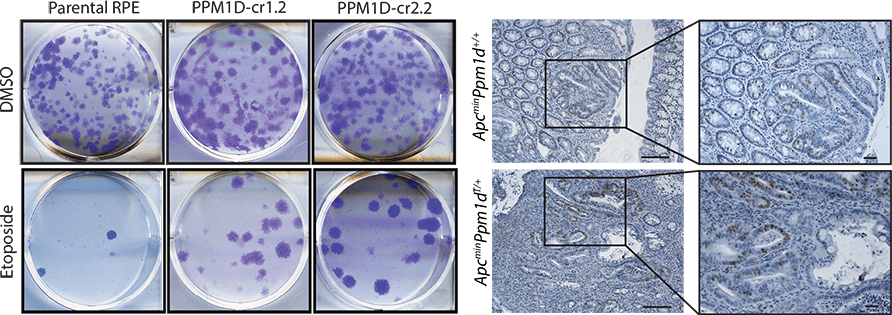
Protein phosphatase PPM1D/Wip1 is an important negative regulator of tumour suppressor p53 and promotes termination of the cell cycle checkpoint. High expression in PPM1D/Wip1 is commonly observed in human tumours, including breast cancer. We have previously identified a new gain-of-function mutation in PPM1D/Wip1 that impairs the cell cycle checkpoints. Using a transgenic mouse model, we have now confirmed the ability of this truncating PPM1D/Wip1 mutation to promote cancer growth. By combining proteomic approaches, biochemistry and cell/molecular biology, we investigate mechanisms of PPM1D/Wip1 function in human cells and seek for its novel targets at chromatin. Finally, we use chemical genetics to evaluate PPM1D/Wip1 as a potential pharmacological target in cancer therapy.
Topic 2. Identification of new cancer-predisposing genes
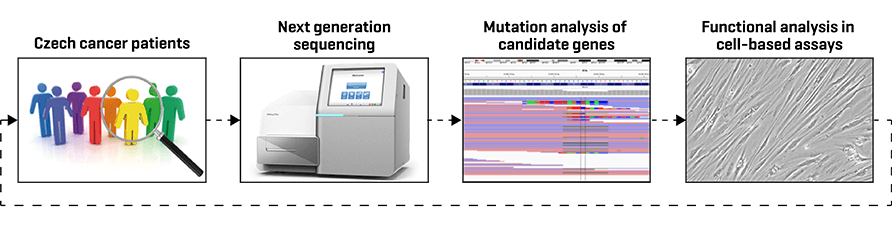
DNA repair and checkpoint genes are typical tumour suppressors that are commonly inactivated in human cancers. When present in the germline, these mutations may increase a risk of cancer development in affected families (such as BRCA1 or CHEK2 in familial breast cancer). Variants of uncertain significance identified by NGS sequencing represent a considerable problem for clinical evaluation. In collaboration with medical geneticists, we develop cell-based assays for functional evaluation of newly identified mutations which will allow better prevention of familial cancers.
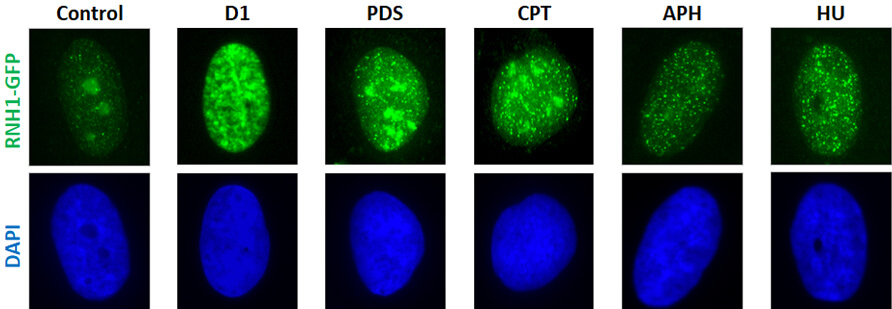
Topic 3. Role of R-loops in genomic instability
R-loops are three-stranded nucleic acid structures generated by invasion of the nascent transcript to the DNA duplex behind the transcription complex. R-loops are emerging as a major source of DNA replication stress, genomic instability and tumour progression. We apply mass spectrometry-based proteomic approaches and functional siRNA screens to identify novel factors involved in the metabolism of R-loops and G4 structures and study their relationship to DNA replication and genome stability. By combining modern molecular/cell biological methods and imaging techniques, we aim to characterize the early events associated with R-loop-mediated replication fork stalling. Finally, we use chromatin immunoprecipitation and next generation sequencing to map the R-loops formed by oncogene-induced replication stress.
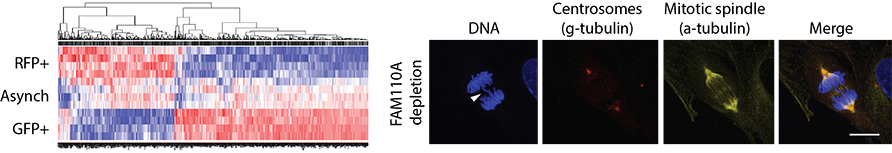
Topic 4. Screening for new proteins involved in the cell cycle and mitosis
Using expression profiling in human non-transformed cells, we have identified putative new regulators of the cell cycle and mitosis. Depletion of FAM110A impaired chromosomal alignment in mitosis and resulted in chromosomal defects. Further analysis revealed that FAM110A localizes at poles of the mitotic spindle and that this localization depends on FAM110A phosphorylation by casein kinase 1. We now follow by investigating new roles of CK1 kinases in mitotic progression.