In a new report in Nature Cell Biology, the laboratory of Petr Svoboda from the Institute of Molecular Genetics (IMG) of the Czech Academy of Sciences, in collaboration with the laboratory of Atsuo Ogura from the RIKEN institute, Japan shows how genome protection in mammalian gametes works.
In a new report in Nature Cell Biology, the laboratory of Petr Svoboda from the Institute of Molecular Genetics (IMG) of the Czech Academy of Sciences, in collaboration with the laboratory of Atsuo Ogura from the RIKEN institute, Japan shows how genome protection in mammalian gametes works.
The stability of the genome, i.e., the complete genetic information of an organism, is threatened by an internal enemy known as transposable elements. These are parasitic DNA sequences that can copy themselves and insert into other places in the host’s genome. New insertions occurring in germ cells and early embryos are then passed on to future generations. Transposable elements thus fundamentally affect the stability and evolution of the genome. For example, they cause growth of the genome size – in humans or mice, the recognizable remains of transposable elements make up about half of their DNA. Sometimes, transposable elements can contribute to evolution in a good way, but more often they damage the genetic information at their insertion site. Therefore, it is not surprising that defense mechanisms against transposable elements. The key player targeting transposable elements in the germline of animals are the so-called piRNAs, short RNA molecules that can recognize active transposable elements and silence them. Based on studies of mouse mutants, mammalian piRNAs were considered essential for the protection of male germ cells but irrelevant in female germ. In contrast, invertebrate and fish piRNAs are also important for normal female fertility. Petr Svoboda’s laboratory now reports that in golden hamsters, piRNAs are indispensable for fertility of both sexes. This fundamentally changes the notion of functioning of the piRNA pathway in mammals and, together with other results, indicates that in this case, the mouse is not a representative model for studying mammalian biology.
It took six years to show that the general assumption for mammals based on mouse experiments was incorrect. “The proposal for a genetic modification of a hamster that would eliminate piRNA protection was created in the spring of 2014, and funding from the European Scientific Council (ERC) was secured from mid-2015,” says Petr Svoboda and he adds: “It turned out to that making a genetic modification in a hamster is much more difficult than what is routinely done in mice. Helena Fulková solved the problem during her internship with Atsuo Ogura, world leader in this field, and she brought hamsters that lacked the piRNA pathway to Prague in 2018. When she started her own laboratory at the Institute of Experimental Medicine of the Czech Academy of Sciences, the analysis was taken over by PhD student Zuzana Loubalová, who completed the project – that’s why Zuzka and Helena share the first authorship,” explains Petr Svoboda.
piRNA in mammals is a timely topic. It is underscored by the fact that the same issue of Nature Cell Biology hosts two other articles reporting analysis of hamsters with mutations in the piRNA pathway. “While each of these articles is different because different components of the piRNA pathway were mutated, they complement each other nicely,” says Petr Svoboda, whose smaller team was able to successfully complete their project in this competition. He says: “The last year was very intense, but we managed. We knew that the Haruhiko Siomi’s group had hamsters with other mutations in the piRNA mechanism, but reached a similar result. We thus coordinated our submissions and left the rest to reviewers, who liked both works in the end. And in the meantime, the third manuscript of colleagues from China appeared. Imagine that – after all those years, costs and work, all three research stories come out together in one day in one issue of one magazine”
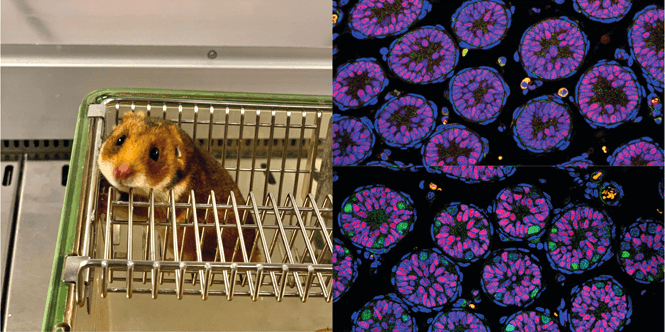
Left: Golden hamster (Mesocricetus auratus)
Right: Sections of testes from thirteen-day-old hamsters. Circular formations are the seminiferous tubules where sperm development will take place in adulthood. In blue is visualized DNA, in red supporting cells, and in green germ cells called spermatogonia, from which sperm will develop in the future. Mutant testes (top right) lack the green-colored spermatogonia that are normally present in the testes (bottom right)
Source: Team of Petr Svoboda (IMG)
Zuzana Loubalova, Helena Fulka, Filip Horvat, Josef Pasulka, Radek Malik, Michiko Hirose, Atsuo Ogura, Petr Svoboda: Formation of spermatogonia and fertile oocytes in hamsters requires piRNAs. Nature Cell Biology. [doi]
Prof. Petr Svoboda, Ph.D.,
phone: (+420) 296 443 147, (+420) 774 798 122,
e-mail: petr.svoboda@img.cas.cz,
web: www.img.cas.cz/research/petr-svoboda/
Martin Jakubec, Ph.D.,
phone: (+420) 296 443 159, e-mail: m.jakubec@img.cas.cz
Full press release as PDF (in Czech).